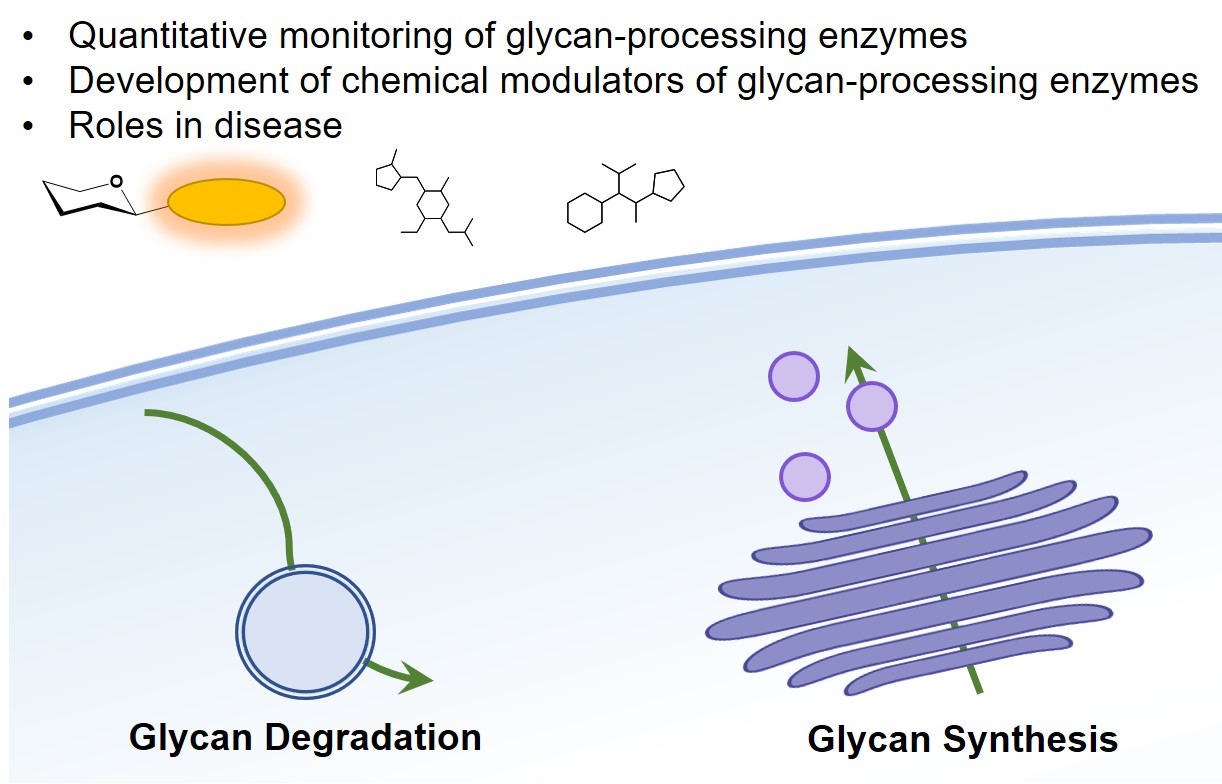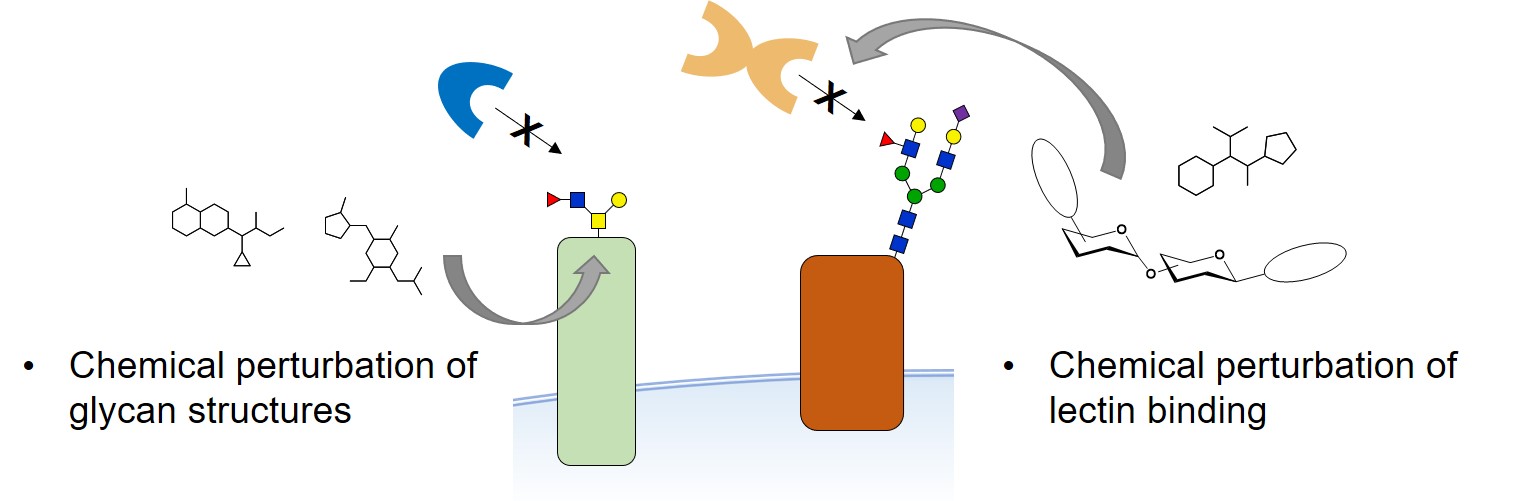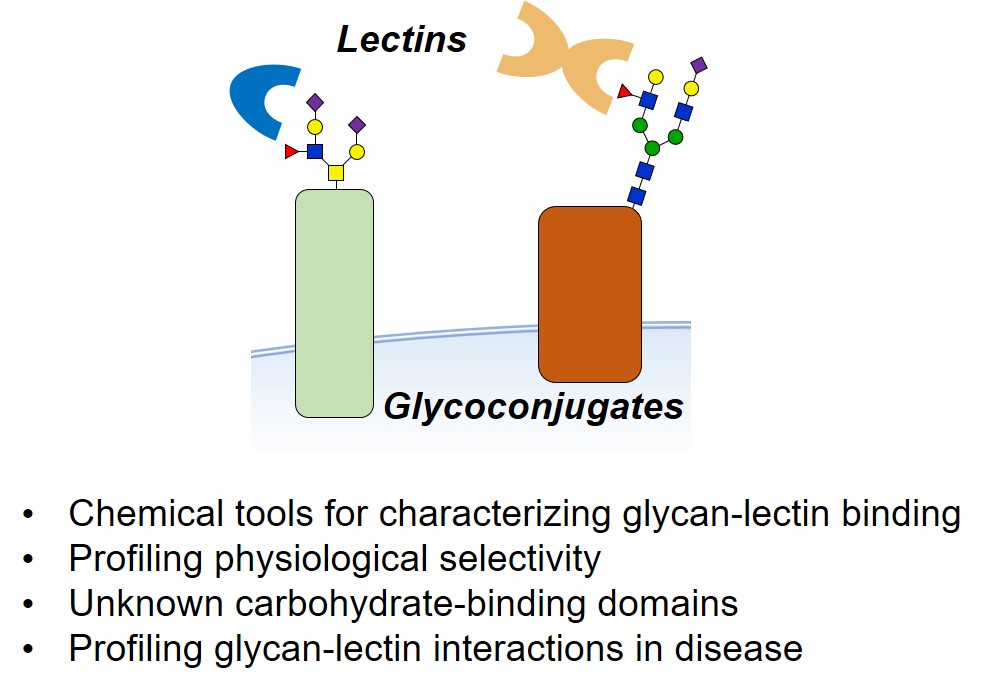
Dissecting glycan-protein interactions in their physiologically relevant environment remains highly challenging. For many important glycan-binding proteins (lectins), endogenous glycoconjugate ligands remain unknown. Furthermore, the existence of yet unknown mammalian glycan-binding domains (GBD) is a topic of high interest.
Current approaches are limited in their ability to capture and to profile glycan-lectin interactions in live cells. Our group develops chemical probes and biochemical strategies that could help address these limitations. We synthesize and exploit proteomic probes as well as photochemical reporters in order to deliver complementary techniques that can be used on cultured cells and in tissues.