Cecioni, S*; Ashmus, R. A. *; Gilormini, P.-A.; Zhu, S.; Chen, X.; Shan, X.; Gros, C.; Deen, M.; Wang, Y.; Britton, R.; Vocadlo, D. J., Quantifying lysosomal glycosidase activity within cells using bis-acetal substrates., Nature Chem. Biol. 2022, 18, 332-341
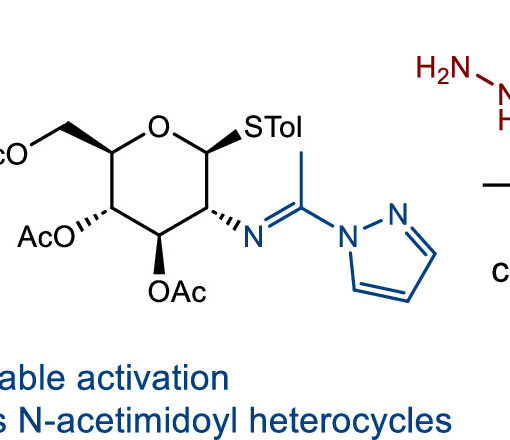
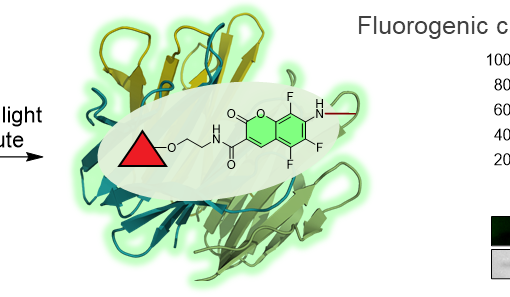
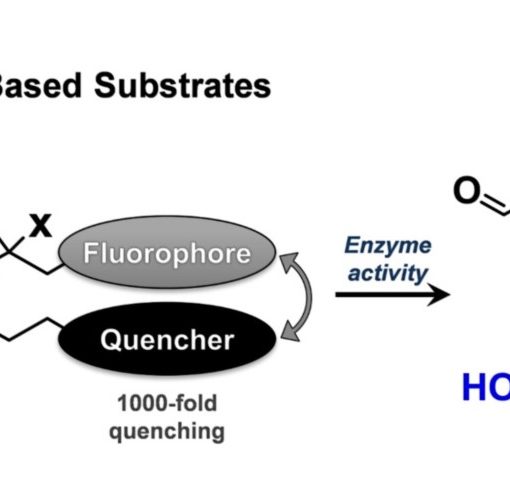
Understanding the function and regulation of enzymes within their physiologically relevant milieu requires quality tools that report on their cellular activities. Here we describe a strategy for glycoside hydrolases that overcomes several limitations in the field, enabling quantitative monitoring of their activities within live cells. We detail the design and synthesis of bright and modularly assembled bis-acetal-based (BAB) fluorescence-quenched substrates, illustrating this strategy for sensitive quantitation of disease-relevant human α-galactosidase and α-N-acetylgalactosaminidase activities. We show that these substrates can be used within live patient cells to precisely measure the engagement of target enzymes by inhibitors and the efficiency of pharmacological chaperones, and highlight the importance of quantifying activity within cells using chemical perturbogens of cellular trafficking and lysosomal homeostasis. These BAB substrates should prove widely useful for interrogating the regulation of glycosidases within cells as well as in facilitating the development of therapeutics and diagnostics for this important class of enzymes.